Mechanisms of Actin Filament Severing and Elongation by Formins
Palmer, N.J., Barrie, K.R., Dominguez, R.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Actin, alpha skeletal muscle | 371 | Oryctolagus cuniculus | Mutation(s): 0 Gene Names: ACTA1, ACTA EC: 3.6.4 | 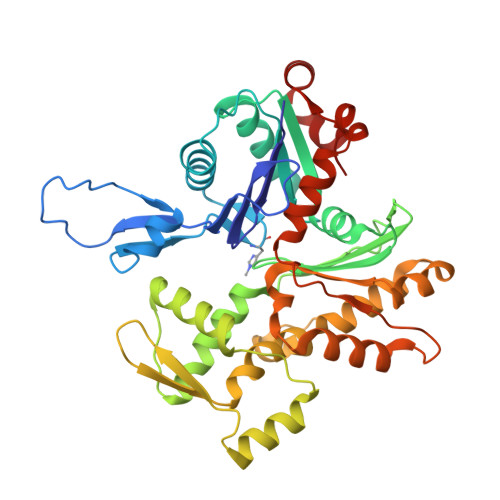 | |
UniProt | |||||
Find proteins for P68135 (Oryctolagus cuniculus) Explore P68135 Go to UniProtKB: P68135 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P68135 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Inverted formin-2 | 60 | Homo sapiens | Mutation(s): 0 Gene Names: INF2, C14orf151, C14orf173 | 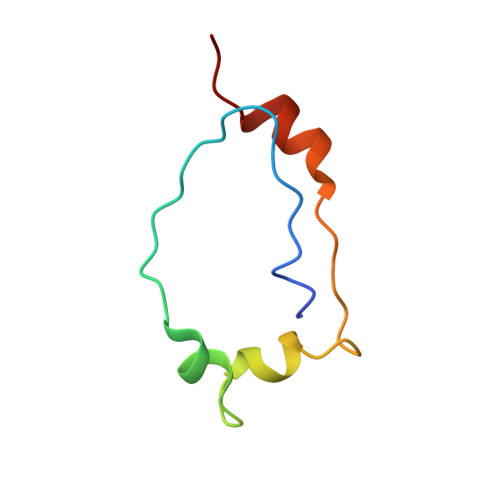 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q27J81 (Homo sapiens) Explore Q27J81 Go to UniProtKB: Q27J81 | |||||
PHAROS: Q27J81 GTEx: ENSG00000203485 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q27J81 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Inverted formin-2 | 323 | Homo sapiens | Mutation(s): 0 Gene Names: INF2, C14orf151, C14orf173 | 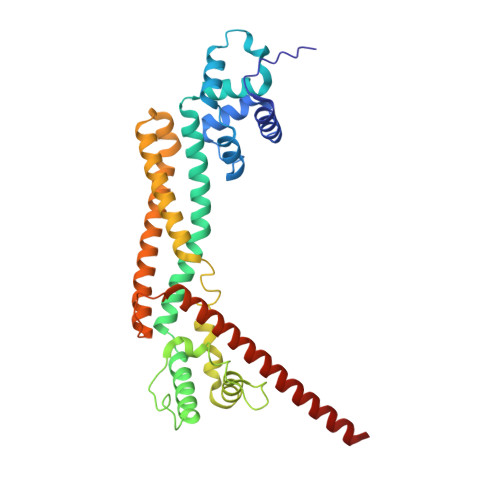 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q27J81 (Homo sapiens) Explore Q27J81 Go to UniProtKB: Q27J81 | |||||
PHAROS: Q27J81 GTEx: ENSG00000203485 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q27J81 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ADP Query on ADP | J [auth A] L [auth B] N [auth C] P [auth D] R [auth E] | ADENOSINE-5'-DIPHOSPHATE C10 H15 N5 O10 P2 XTWYTFMLZFPYCI-KQYNXXCUSA-N |  | ||
| MG Query on MG | K [auth A] M [auth B] O [auth C] Q [auth D] S [auth E] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| HIC Query on HIC | A, B, C, D, E A, B, C, D, E, F, I | L-PEPTIDE LINKING | C7 H11 N3 O2 |  | HIS |
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | cryoSPARC | 4.0 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | -- |