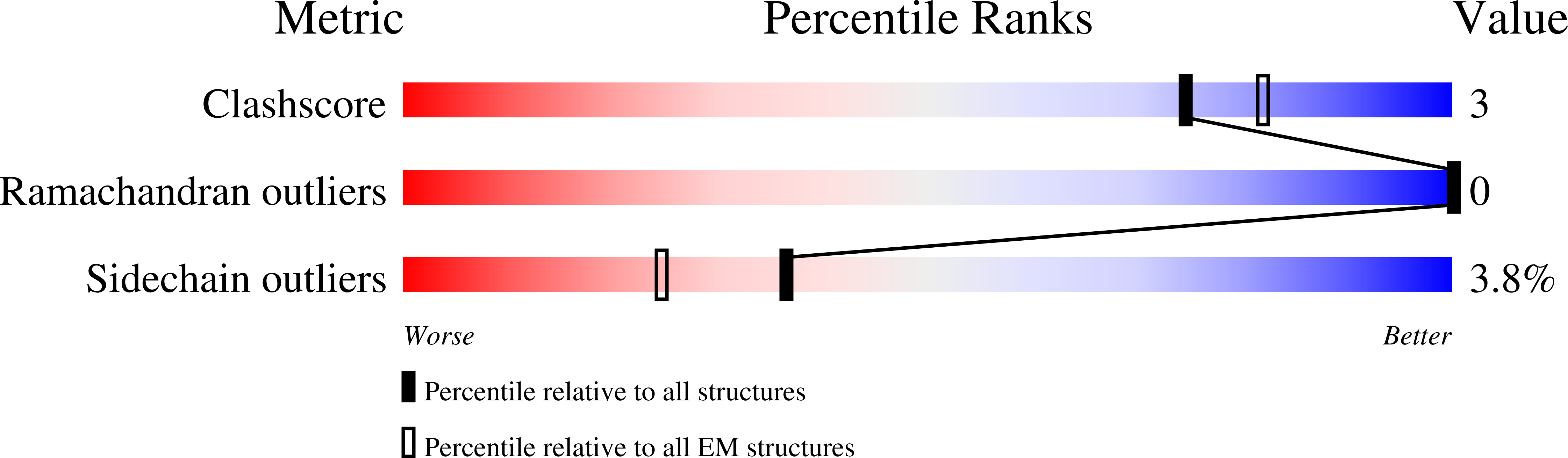
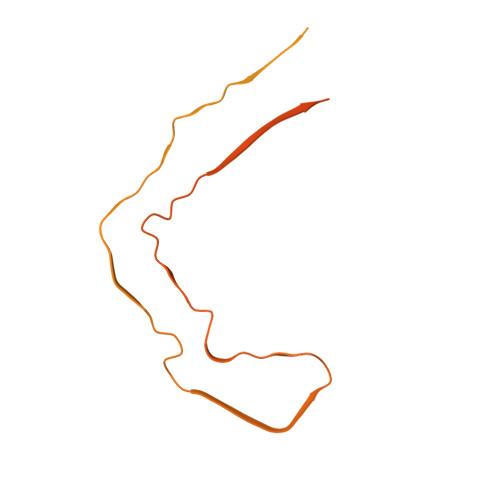
Tau filaments with the chronic traumatic encephalopathy fold in a case of vacuolar tauopathy with VCP mutation D395G.
Qi, C., Kobayashi, R., Kawakatsu, S., Kametani, F., Scheres, S.H.W., Goedert, M., Hasegawa, M.(2024) Acta Neuropathol 147: 86-86
- PubMed: 38758288
- DOI: https://doi.org/10.1007/s00401-024-02741-x
- Primary Citation of Related Structures:
9ERM, 9ERN, 9ERO - PubMed Abstract:
Dominantly inherited mutation D395G in the gene encoding valosin-containing protein causes vacuolar tauopathy, a type of behavioural-variant frontotemporal dementia, with marked vacuolation and abundant filamentous tau inclusions made of all six brain isoforms. Here we report that tau inclusions were concentrated in layers II/III of the frontotemporal cortex in a case of vacuolar tauopathy. By electron cryomicroscopy, tau filaments had the chronic traumatic encephalopathy (CTE) fold. Tau inclusions of vacuolar tauopathy share this cortical location and the tau fold with CTE, subacute sclerosing panencephalitis and amyotrophic lateral sclerosis/parkinsonism-dementia complex, which are believed to be environmentally induced. Vacuolar tauopathy is the first inherited disease with the CTE tau fold.
Organizational Affiliation:
Medical Research Council Laboratory of Molecular Biology, Cambridge, UK.